Abstract
Introduction: While the chemoimmunotherapy combination of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) is the standard-of-care in 1L treatment for DLBCL, 30-40% of patients either relapse or are refractory to R-CHOP (Coiffier et al. N Engl J Med 2002). The initiation of subsequent therapies post relapse is integral to relapsed/refractory (R/R) DLBCL management and is associated with substantial treatment cost. Previous studies of treatment costs for R/R DLBCL patients are outdated and exclude novel therapies (e.g. chimeric antigen receptor [CAR-]T therapies). Thus, the cost of disease progression may be underestimated. Using data through 2020, we sought to understand the economic burden associated with disease progression in the DLBCL treatment landscape.
Methods: This was a retrospective cohort study using administrative claims data from IQVIA PharMetrics ® Plus (a US commercial claims database). We identified patients ≥18 years, who received 1L R-CHOP treatments between January 1, 2010 and June 30, 2018. Patients were required to have ≥1 inpatient claim or ≥2 outpatient claims with a DLBCL diagnosis from 1 year before to 90 days after initiating R-CHOP. End of therapy was defined as a gap of ≥60 days in the treatment regimen or initiation of new agents (OPTUM 2018). Using this definition as a proxy for progression, patients not receiving second-line (2L) treatment for ≥2 years were assigned to the 'no progression' cohort and those who initiated non-R-CHOP therapy after 1L therapy to the 'progression' cohort. In both cohorts, index date was defined as either 60 days after the end of 1L treatment, or the initiation of 2L treatment, whichever occurred first. All patients had continuous enrollment in medical and pharmacy benefits between R-CHOP initiation and ≥2 years post index date, allowing time for post-1L relapse. Costs per-patient-per-month (PPPM) and 3-year cumulative all-cause costs (2020 USD) were compared between cohorts. Generalized linear models (gamma distribution with log link) were performed to adjust for baseline characteristics, including age and payer type at index, sex, US region, Charlson Comorbidity Index (CCI), and baseline total cost of care 1 year before index date.
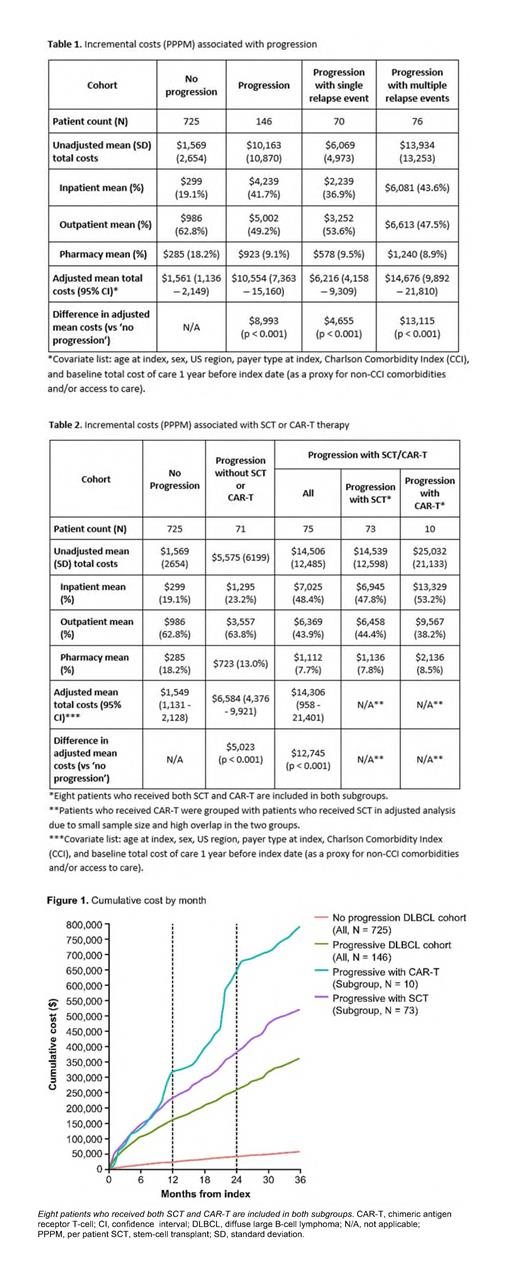
Results: Overall, 871 patients were identified; 58% were female. The mean (standard deviation [SD]) age and CCI (excluding malignancy) at index date were 58.0 (11.7) years, and 2.5 (3.1) years, respectively. The mean follow-up period was 45 months. Patients in the 'no progression' cohort (N = 725; 83.2%) had similar baseline characteristics to those in the 'progression' cohort (N = 146; 16.8%). About half (N = 76; 52.1%) of the progression cohort had multiple relapse events, with 30.8% receiving ≥4 lines of therapy. Among the progression cohort, 73 (50.0%) and 10 (6.8%) patients received stem-cell transplant (SCT) and CAR-T therapy, respectively.
The mean PPPM cost was higher among progressors than non-progressors (unadjusted: $10,163 vs $1,569; adjusted: $10,554 vs $1,561, p < 0.001, Table 1). The major cost driver for disease progression was inpatient costs (41.7% of total costs for progressors vs 19.1% for non-progressors). Cost of progression associated with multiple relapse events was more than twice the cost associated with a single event (unadjusted: $13,934 vs $6,069; adjusted: $14,676 vs $6,216, p < 0.001, Table 1). Compared with no progression, receiving novel treatments after 1L was associated with considerably higher costs (unadjusted: $14,539 [SCT] and $25,032 [CAR-T] vs $1,569; adjusted: $14,306 [SCT/CAR-T] vs $1,549, p < 0.001, Table 2). Similar trends were seen comparing unadjusted 3-year cumulative costs based on month of disease progression in patients in the 'no progression', 'progression' and SCT/CAR-T subgroups (Figure 1).
Conclusion: In this analysis of insurance claims through 2020, we systematically selected patients who survived for at least 2 years after the index date; this represented approximately 80% of DLBCL patients treated with 1L R-CHOP. The cost of disease progression in DLBCL is considerable, especially among patients receiving novel treatments as later lines of therapy. The costs for patients experiencing multiple relapse events were more than twice the cost for patients with a single relapse event. Effective frontline treatments for DLBCL are needed to reduce the economic burden associated with disease progression.
Wang: Aurinia Pharmaceuticals Inc.: Current equity holder in publicly-traded company; Novavax, Inc.: Current equity holder in publicly-traded company; Oragenics, Inc.: Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; The SPHERE Institute: Ended employment in the past 24 months; Genentech, Inc.: Current Employment; TG Therapeutics, Inc.: Current equity holder in publicly-traded company. Roth: Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company; Seattle Genetics: Consultancy; Bayer Healthcare: Consultancy; Bristol-Myers Squibb: Consultancy; Epigenomics AG: Consultancy. Ng: Genentech, Inc./F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company; Emory University, Rollins School of Public Health: Ended employment in the past 24 months. Hossain: Genentech, Inc.: Current Employment; Legend Biotech: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Li: F. Hoffmann-La Roche Ltd/Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Masaquel: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal